Nitric Acid Production Line (100,000-270,000 t/y)
Complete nitric acid production system for nitric acid using ammonia oxidation process and applicable to fertilizer manufacturing
Nitric acid is predominantly produced using the ammonia oxidation process.
Based on the ammonia oxidation pressure and the absorption pressure of nitrogen oxides, dilute nitric acid production technologies can be classified into the following five routes: Atmospheric-pressure process, combined-pressure process, medium-pressure process, high-pressure process, dual-pressure process.
- Plant capacity: 100,000 t/y, 150,000 t/y, 200,000 t/y, 270,000 t/y
- Product quality: Complies with GB/T 337.2-2014 Industrial Nitric Acid: Dilute Nitric Acid
| Item | Atmospheric-pressure process | Combined-pressure process | Medium-pressure process | High-pressure process | Dual-pressure process |
| Oxidation pressure/MPa(a) | 0.11-0.12 | 0.1 | 0.4-0.6 | 0.8-1.3 | 0.4-0.6 |
| Absorption pressure/MPa(a) | 0.98-1.8 | 0.4-0.6 | 0.4-0.6 | 0.8-1.3 | 0.8-1.2 |
| Product concentration/% | 40-45 | ≤50 | ≤53 | ≥60 | ≥60 |
| Ammonia consumption/kg/t | 308-330 | 280 | 282-284 | 288-300 | 282-284 |
| Platinum consumption/mg/t | 60 | 60-80 | 120 | 200 | 120 |
| Power consumption/kWh/t | / | / | 10 | 25 | 11.1 |
| NOₓ concentration at absorber outlet off-gas/×10⁻⁶ | 5000-10000 | 2500 | 1000-1500 | 2000-2500 | 200-800 |
| NOₓ concentration after off-gas treatment/×10⁻⁶ | Atmospheric alkali absorption: 600-1300 | Atmospheric alkali absorption: 400-600 | Pressurized alkali absorption: 200-500 | ≤200 | ≤200 |
| Cooling water/ΔT = 10℃ | / | / | 170 | 176 | 160 |
| By-product steam/t/t | / | / | 0.18 | 0.3 | 0.301 |
| Ammonia oxidation efficiency/% | / | / | 96 | 94 | 96.6 |
| NOₓ absorption efficiency/% | / | / | 98 | 99.6 | 99.8 |
Notes:
Power consumption does not include electricity for lighting, ventilation, or heating.
For the full high-pressure process, power consumption includes electricity used by the refrigeration system.
Based on the comparison table above:
-
Ammonia and platinum consumption:
The combined-pressure process shows the lowest consumption, followed by the medium-pressure and dual-pressure processes, while the high-pressure process has the highest consumption. -
Capital investment:
The high-pressure process requires the lowest investment, followed by the dual-pressure process. -
Plant capacity and scalability:
The high-pressure process and the dual-pressure process are the most suitable for large-scale plant implementation. -
Off-gas emissions:
The dual-pressure process offers the best off-gas emission performance. -
Conclusion:
The dual-pressure process is currently the most advanced nitric acid production technology.
- High ammonia oxidation efficiency (96.6%) with low platinum consumption. (120mg/t of 100 wt% HNO₃ before platinum recovery)
- High nitrogen dioxide absorption efficiency (99.8%), enabling nitric acid concentrations of up to 60%.
- Very low NOₓ concentration in off-gas after ammonia reduction treatment (≤ 50 ppm).
- Recovery of medium-temperature off-gas energy; optimal matching between the steam turbine and off-gas expansion turbine, allowing recovery of approximately 60% of the compression power.
- Efficient utilization of the cooling capacity generated during low-pressure ammonia evaporation to produce low-temperature cooling water for absorption tower cooling.
- Effective utilization of the polytropic compression energy of air compressors and NOₓ compressors within the plant.
- By-product steam can be exported in addition to driving compressor units, resulting in low specific power consumption (11.1kWh/t of 100 wt% HNO₃)
- Adoption of a DCS control system, providing safer operation, improved automation, and easier process control.
-
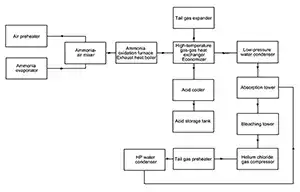
Ammonia-air mixture preparation
Liquid ammonia is fed into ammonia evaporators (A and B), where it is vaporized to form gaseous ammonia. The gaseous ammonia is then superheated to above 100℃ in a heater using steam, and subsequently passes through an ammonia gas filter to remove oil and impurities. The purified ammonia gas is delivered to the ammonia-air mixer, where it is mixed with primary air compressed to 0.35MPa(g) and heated to approximately 236℃. The ammonia concentration is controlled at 9.6%, and the stable ammonia-air mixture is fed to the oxidation reactor. -
Ammonia oxidation and waste heat recovery
The ammonia-air mixture enters the top of the oxidation reactor and is evenly distributed onto the platinum gauze catalyst by a distributor. Catalytic oxidation of ammonia takes place according to the reaction: 4NH₃ + 5O₂ → 4NO + 6H₂O + Q
A large amount of heat is released, and the reaction temperature is controlled at approximately 860℃. The reaction products pass through a steam superheater and a waste heat boiler, where superheated steam at 3.9MPa and 440℃ is generated. This steam is supplied to the steam turbine of the four-in-one unit, while excess steam is exported to the external steam network. -
NO oxidation and heat recovery
Nitrogen oxide gas leaving the waste heat boiler passes through a high-temperature gas-gas heat exchanger and an economizer, then enters a low-pressure reaction water cooler, where the NO gas is cooled to approximately 42℃. Simultaneously, NO is oxidized to NO₂ in equipment and piping: 2NO + O₂ → 2NO₂ + Q
NO₂ reacts with condensed steam to form dilute nitric acid. The acid-gas mixture enters a gas-liquid separator, where dilute nitric acid is separated and sent to the absorption tower. The remaining NO gas is mixed with secondary air from the bleaching tower and fed to the nitrogen oxides compressor, where it is compressed to 1.0MPa(g).
During compression, the temperature rises from 42℃ to approximately 200℃. The compression heat is removed in an off-gas preheater, preheating the off-gas, while the NO gas temperature is reduced to about 130℃. The NO gas is then cooled to approximately 40℃ in a high-pressure reaction water cooler and fed to the bottom of the absorption tower, where the condensed acid mixes with the product acid. -
NOₓ absorption and nitric acid bleaching
Nitrogen oxides are absorbed by water in the absorption tower to produce 60-65 wt.% nitric acid. The dilute nitric acid is sent to the bleaching tower, where it is bleached by secondary air and then transferred to the product acid storage tank. The main absorption reaction is: 3NO₂ + H₂O → 2HNO₃ + NO + 142kcal/kg -
Off-gas energy recovery and discharge
The off-gas exits from the top of the absorption tower and enters an off-gas separator, where liquid droplets are removed. The gas is first heated to approximately 55℃ in a secondary air cooler, then further heated to about 155℃ in the off-gas preheater by high-pressure nitrogen oxide gas. Finally, the off-gas is heated to 360-390℃ in a high-temperature gas-gas heat exchanger and sent to an off-gas expansion turbine, where work is recovered. More than 50% of the total compression power can be recovered through this energy recovery system. After catalytic ammonia reduction treatment, the NOₓ concentration in the off-gas is reduced to ≤ 56ppm(v), and the treated off-gas is discharged via the off-gas stack.
- Four-in-one unit: Including steam turbine, nitrogen oxides compressor, air compressor, off-gas expansion turbine, gearbox and auxiliary equipment.
- Ammonia oxidation reactor: An integrated unit consisting of:
- Upper section: multi-layer perforated baffle distributor
- Middle section: basket-type platinum gauze holder
- Lower section: steam superheating zone and waste heat boiler with furnace-wall cooling coils
- Absorption tower: Double-S type, single-overflow sieve tray tower. 32 trays in total, including 26 trays equipped with cooling coils
- Bleaching tower: Sieve tray tower with 4 trays
Delandi (Nantong) Machinery is dedicated to the development of the fertilizer industry, focusing on process design, production equipment manufacturing, and production performance improvement for fertilizer manufacturing projects. We bring together a team of experienced professionals with deep technical knowledge and long-term industry involvement.